NephroPlex™ IgA Nephropathy Immune Activity Assessment
Human Serum/Plasma Poly-IgA Immune Complex Assay Kit (ELISA)
Product Introduction
NephroPlex™ kit, developed by Shenzhen Luwei Biotechnology Limited, is based on exclusively patented molecular probe technology to detect circulated poly-IgA immune complex in the peripheral blood. The renal deposition of Poly-IgA immune complex is mainly responsible for the pathological onset of IgA nephropathy. Experiments have verified that this indicator can be an excellent indicator for the assessment of immune activity of patients with IgA nephropathy.
As a first-in-class specific molecular biomarker of IgA nephropathy in the peripheral blood, Human Serum/Plasma Poly-IgA Immune Complex Assay Kit (ELISA) will lead a major breakthrough in the immune activity monitoring, companion diagnosis, fast turnaround treatment assessment, and life cycle management of patients with diagnosed IgA nephropathy; and early stage of IgA nephropathy diagnosis and risk assessment for patience with abnormal renal activities such as proteinuria and hematuria.
The relevant validation experiments are as follows.
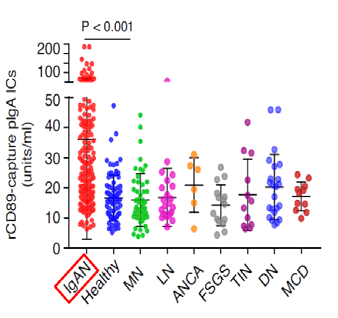
Experiment 1
IgAN patients has much higher levels of IgA immune activity index than healthy control and other glomerulonephritis patients.
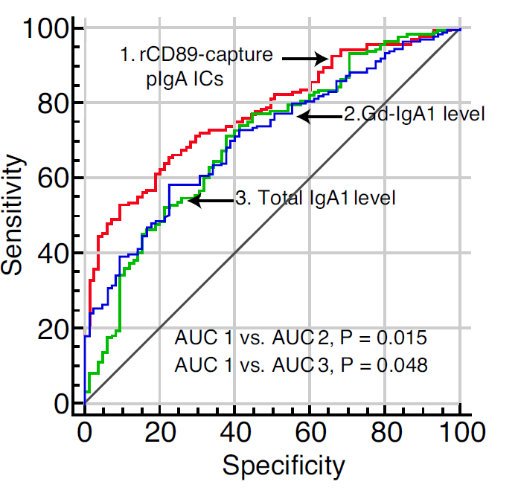
Experiment 2
CD89-captured Poly-IgA complex Assay, compared to traditional biomarkers (total IgA1 and Gd-IgA1), shows better specificity and sensitivity with the highest AUC.
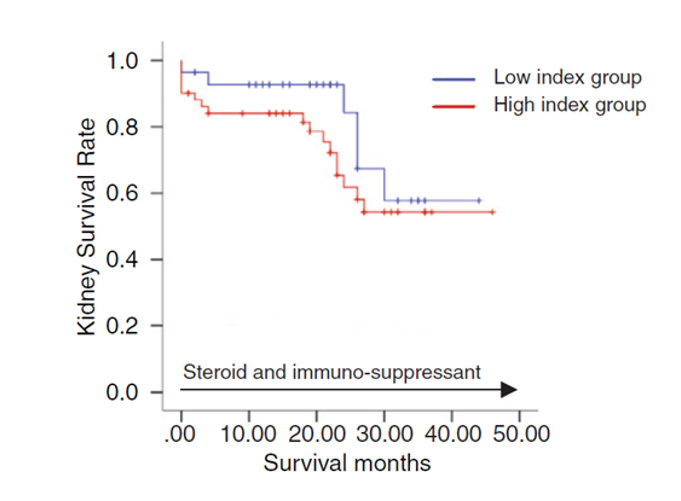
Experiment 3
Comparison of survival curves of diagnosed patients with high/low IgA immune activity index (K-M curve).
The detection advantage
Simple and Fast (only a small amount of blood draw).
Verified assay stability, repeatability and accuracy.
Clinical application value
1. Early diagnosis
For unpunctured patients with abnormal urine protein or blood in the urine, a high index of immune activity predicts a high risk of IgA nephropathy.
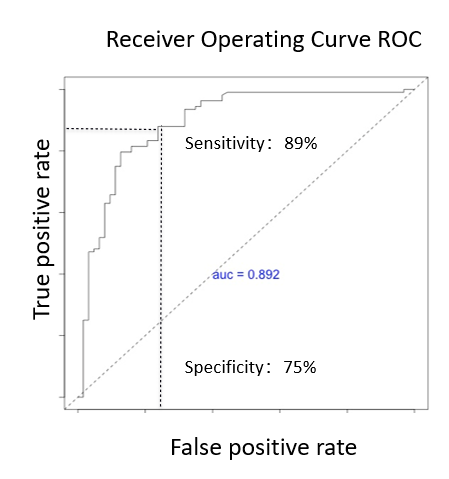
- IgAN patients diagnosed via renal biopsy vs healthy control cohort
- 108 IgAN patients vs 63 healthy control
- AUC = 0.89
- Specificity: 75%
- Sensitivity: 89%
2. Efficacy assessment
Efficacy evaluation of IgA nephropathy may give faster sign of improvement than the traditional clinical variables such as eGFR, and provide valuable and timely feedback to physicians and patients.
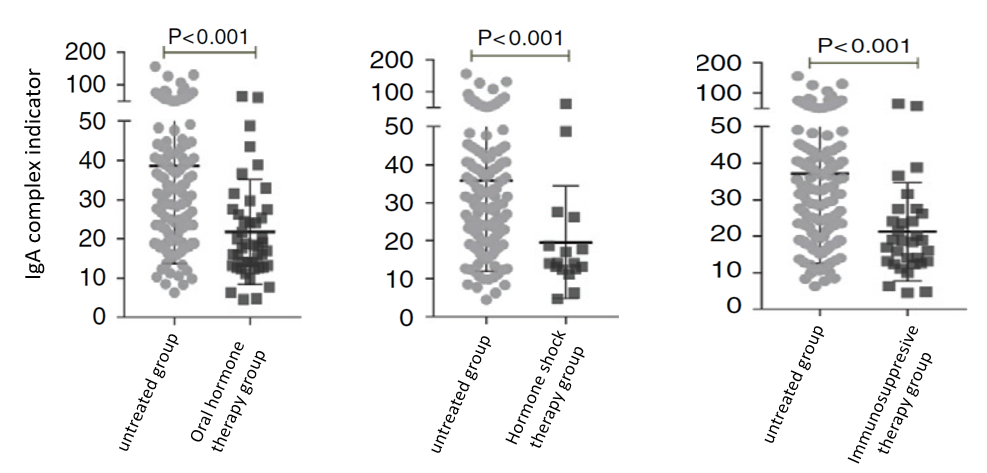
Effective treatment significantly reduces the indicators of IgA complex